UPDATE: A Spark of HOPE Amidst a World of Uncertainty
COVID-19 Vaccine Clinical Trial
4/16/2020 Update
In the oncology community, the response to COVID-19 has been swift to protect healthcare providers and cancer patients. Our patients may be more vulnerable to contracting the virus due to immunosuppression caused by malignancy and anticancer therapies.
A recent study conducted in Wuhan, China analyzed 18 confirmed cases of COVID-19 in patients with cancer. Results from the study revealed that this population is at higher risk for experiencing severe complications. The study concluded that cancer patients receiving antitumor treatments should undergo vigorous screening for COVID-19 infection and try to avoid treatments that cause immunosuppression when possible.
Researchers around the world are working diligently to develop and implement clinical trials to help us not only prevent and treat COVID-19, but also understand more about the respiratory illness. Today, over 400 COVID-19 related clinical trials are in development globally, with more than 250 research trials actively recruiting patients.
Similar to cancer, COVID-19 can affect us all – every country, every race, every gender and every age group. It is only through clinical trials that we will learn how to safely and effectively combat this virus.
Illinois CancerCare is committed to bringing the most advanced treatment options to our patients through clinical trials. Our team is actively reviewing both cancer and COVID-19 related clinical trials to ensure that our patients have access to groundbreaking research close to home.
Learn more about clinical trials and to stay up to date on what trials we currently have available by clicking here.
3/19/2020 Update
Clinical trial testing of the first potential vaccine to prevent COVID-19 began on 3/16/2020 in Seattle, Washington. COVID-19, also referred to as the Coronavirus, is a respiratory illness that can spread from person to person. This contagious virus spreads through the transmission of respiratory droplets produced when an infected individual coughs or sneezes. These infectious droplets can be inhaled by anyone in close proximity or possibly transferred by touching a surface that contains the viral droplets and then touching your eyes, nose or mouth. With COVID-19 diagnoses and related deaths increasing each day, treatment and prevention are needed immediately!
Scientists across the country are working around the clock to develop and evaluate therapies that can put a stop to this pandemic. Moderna Inc., a clinical stage biotechnology company, has developed a new vaccine that is now undergoing human testing in a phase 1 clinical trial at the Kaiser Permanente Washington Health Research Institute in Seattle, Washington. Does this mean that a vaccine will be available next week or next month? Unfortunately, the answer is no.
Phase 1 clinical trials are just the first step of a three-part process leading to FDA approval. Each phase of a clinical trial is important to ensure that all drugs are properly evaluated for safety and efficacy in humans prior to approval for public use.
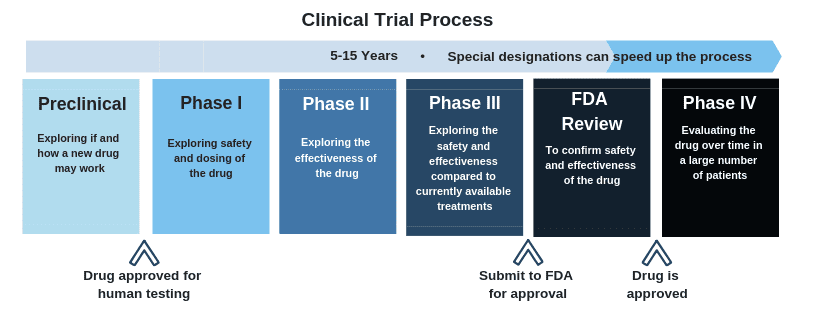
On average, clinical trial evaluation of a novel vaccine or treatment can take several years. Increased clinical trial participation can help expedite this process! “Finding a safe and effective vaccine to prevent infection from the new coronavirus is an urgent public health priority,” Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, said in a statement Monday. “This phase 1 study, launched in record speed, is an important first step toward achieving that goal.” A vaccine is not expected to be available for public use for 12-18 months as it proceeds through the clinical trial process.
This early-phase clinical trial is led by Dr. Lisa Jackson, a senior investigator at Kaiser. Study participants will receive two doses of the vaccine via an injection into the muscle of the upper arm, approximately 28 days apart. Each participant will be assigned to receive a 25 microgram (mcg), 100 mcg or 250 mcg dose at both vaccinations. 45 patients, between the ages of 18 and 55, will be enrolled on this phase 1 clinical trial. Only adults that reside in the Seattle, Washington area are eligible. If the data from this phase 1 clinical trial yields positive results, the vaccine will be approved by the FDA to move forward to phase 2 clinical trial testing.
Until a treatment or vaccine is available, everyone has a responsibility to do their part to minimize transmission of this virus. The Centers for Disease Control and Prevention has the following recommendations:
- Wash hands often with soap and water for at least 20 seconds
- Utilize hand sanitizer containing at least 60% alcohol when soap and water are not readily available
- AVOID touching your eyes, nose, and mouth with unwashed hands
- Cover your mouth and nose with a tissue when you cough or sneeze or use the inside of your elbow.
- Practice social distancing whenever possible by maintaining at least 6 feet of distance from the next person
- STAY HOME if you are sick. Seek medical care if needed.
- Clean and disinfect frequently touched surfaces daily.
To learn more about this clinical trial, click here.
For a quick review on the four phases of the clinical trial process, check out our research education video here.




