What are Clinical Trials?
A clinical trial is a research study that tests how well a new medical approach works in people. As our patient, you may have an opportunity to be a part of a clinical trial utilizing cutting edge treatment options. Participating in a clinical trial is 100% voluntary. Taking part in research has the potential to benefit not only you, but future cancer patients as well. Prior to making a decision to enroll in a clinical trial, a research professional and your physician will sit down with you and your family/friends to discuss the study in detail and answer any questions you may have. We encourage our patients to take the time needed to make an informed decision regarding participation.
Cancer research encompasses much more than cancer treatment. This life-changing research focuses on each part of a patient’s journey starting with screening and continuing through survivorship. Clinical oncology research today includes clinical trials dedicated to cancer prevention, screening, diagnosis, symptom management, cancer care delivery, and survivorship.
The clinical trials offered at Illinois CancerCare are hand selected by our physicians and undergo an extensive review process to ensure that our trial menu accurately reflects our patient population and offers the most advanced treatment options available. A full listing of our current clinical trials are available here. Be sure to speak with your oncologist about what trials may be a great option for you, at your next visit.
Phases of Clinical Trials
Most clinical research that involves the testing of a new drug or treatment progresses in a series of steps, called phases. These phases allow researchers to ask and answer questions in a way that results in reliable information about the drug and protects the patients. Clinical trials are generally classified into one of four phases. If a new treatment is successful in one phase, it can be approved by the Food and Drug Administration (FDA) to move on to the next. All new treatments undergo years of rigorous laboratory testing prior to being offered to humans on a clinical trial. Due to stringent safety guidelines put forth by the FDA, less than 3% of all newly developed therapies advance forward to clinical trial testing.
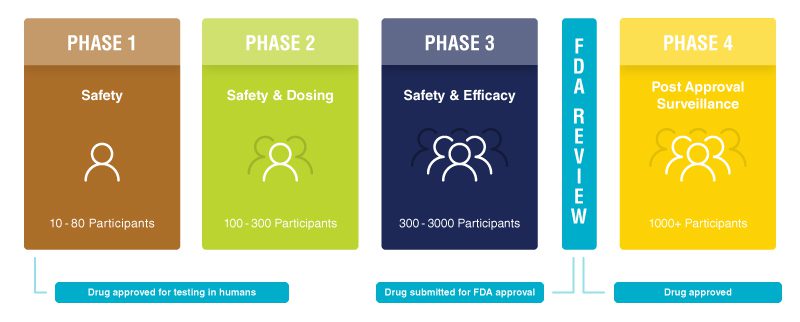
- Phase I trials: These initial studies evaluate how a new drug should be given (by mouth, injected into the blood, or injected into the muscle), how often, and what dose is safe. A phase I trial usually enrolls only a small number of patients.
- Phase II trials: A phase II trial continues to test the safety of the drug, and begins to evaluate how well the new drug works. Phase II studies usually focus on a particular type of cancer.
- Phase III trials: These studies test a new drug, a new combination of drugs, or a new surgical procedure in comparison to the current standard. A participant will usually be assigned to the standard group or the new group at random (called randomization). Phase III trials often enroll large numbers of people and may be conducted at many clinics and cancer centers nationwide. A new treatment is submitted to the FDA for review and possible approval following the completion of the phase III trial.
- Phase IV trials: These trials assess drugs that have already been approved by the FDA and are available for patients. Phase IV trials generally include thousands of participants and assess the long term effectiveness and safety of a treatment. They may also analyze other aspects of treatment such as quality of life or cost effectiveness.



