Journal of American Medical Association (JAMA) Publication
Dr. Pankaj Kumar – Published in Journal of American Medical Association (JAMA) – the most widely circulated general medical journal of peer-reviewed articles in the world.
Congratulations to Dr. Pankaj Kumar of Illinois CancerCare and the Heartland NCI Community Oncology Research Program on this Achievement!
Effect of Celecoxib vs Placebo Added to Standard Adjuvant Therapy on disease-Free Survival Among Patients With Stage III Colon Cancer.
Investigation
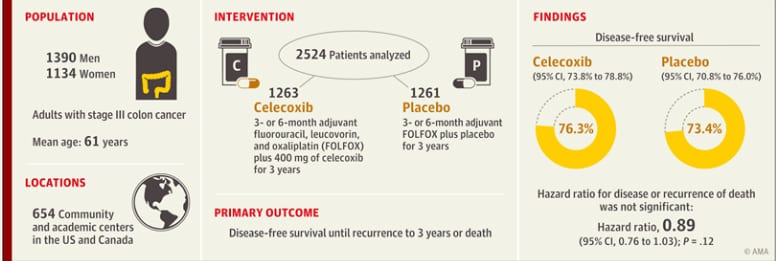
In Phase III clinical trials, investigational drugs are often added to the standard of care to determine if additional benefit ca be gained. This study added an anti-inflammatory drug, celecoxib, to the standard treatment for stage III colon cancer. The hope was that the addition of this drug would decrease the change of the cancer returning (recurrence).
Findings
The addition of celecoxib to standard chemotherapy did not significantly reduce the risk of a patient’s cancer returning.
Illinois CancerCare was 1 of 654 community and academic centers throughout the United States and Canada to offer clinical trial CALGB 80702 to their patients. This large, phase III trial enrolled over 2,500 participants!
Question
Does the cuclooxygenase 2 inhibitor celecoxib, when added to standard adjuvant chemotharapy, improve disease-free survival among patients with stage III colon cancer?
Conclusion
This clinical trial found that in patients with stage III colon cancer, the addition of celecoxib, compared with placebo, to standard adjuvant chemotherapy did not significantly improve disease-free survival
Meyerhardt JA, Shi Q, Fuchs CS, et al. effect of celecoxib vs placebo added to standard adjuvant on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA. Published April 6, 2021. doi: 10.1001/jama.2021.2454



